Introduction
Much is unknown about the cause of post-traumatic headaches (PTHA). Opinions on how brain trauma produces headaches are based substantially on theoretical conclusions drawn from what is known, and often likewise theorized, about other types of more common headaches. Similar processes are no doubt involved with the same pain pathways in play, but fundamentally, the root origins and cellular-level explanations remain for the most part a mystery.
This article will highlight the characteristics of PTHA (such as they are), the pathophysiological origins of PTHA (such as are known), and the array of treatment options available through a multidisciplinary approach. In writing this article every effort was made to use basic, understandable terminology. With all apologies to the reader, certain topics are difficult to synthesize to simplicity. This is one of them. Medical terminology at times can be its own foreign language.
Definitions and Prevalence
A post-traumatic headache is a symptom of traumatic brain injury (TBI), not an independent condition, and is considered the most common and most misunderstood. 1 PTHA can result from the full spectrum of brain injury classifications: mild, moderate, and severe. It is defined by the International Headache Society as “a headache that develops within seven days after head trauma or after regaining consciousness.”
Post-traumatic headaches are regarded as chronic (CPTHA) when they continue for more than 2 months from the occurrence of injury, although a duration of 6 months has been suggested. 2,3
The overall incidence of TBI in the United States is a staggering 1.8 million cases per year, of which 30 to 90 percent, per retrospective studies, will have a post-traumatic headache component. Fully 2 percent of the entire U.S. population is disabled secondary to post-traumatic headaches. In addition, nearly 45 percent of head and neck injuries are accompanied by chronic headaches at six months, and at 1 year, headache pain is present in 20 percent of such individuals and is considered chronic.
Despite the prevalence of TBI and its potential long-term effects, at least 25 percent of people with mild traumatic brain injury (MTBI) seek no medical attention whatsoever, compared with 14 percent who will visit their primary care physician, physician’s office or clinic following a head injury. For those who seek treatment typically a computed tomography scan (CT Scan) is initially performed (emergency room setting). And later, if TBI symptoms persist, a magnetic resonance imaging scan (MRI) will likely be done. Neither of these studies can predict whether post-traumatic headaches will develop in a patient. No test is predictive of PTHA. Likewise, no testing exists that can predict whether a post-traumatic headache will ultimately become intractable and develop into a lasting, chronic post-traumatic headache (CPTHA).4
Headache Classifications and Characteristics of Post-Traumatic Headaches
Headaches are of different types and broadly classified into 2 categories: primary and secondary. Primary headaches are not the result of another condition, whereas secondary headaches are. As a secondary headache, PTHA is thought to have a structural or physiological basis that, if corrected, results in the resolution of the headache.5
Primary headaches include migraine, tension, and cluster headaches and are thought to be genetically acquired syndromes that involve trigeminovascular pathway dysfunction.6 These headache types have their own unique characteristics and are not caused by traumatic brain injury. Secondary headaches are headaches resulting from underlying conditions such as brain infection (encephalitis, meningitis, abscess), brain tumor, hydrocephalus (excess water on the brain), high blood pressure (hypertension), problems with blood vessels (hemorrhage), neck injury, and traumatic brain injury.
It is important to note that TBI-related headaches mimic the characteristics of migraines and are often referred to as migraines by treating clinicians despite the fact such headaches are of completely different origins. Traumatic brain-injury related headaches have no unique defining characteristics of their own. Because the symptom characteristics are like primary headaches, treatments for PTHA are currently the same as those used for migraine and tension headaches. PTHA and CPTHA symptoms can therefore include:
- Dull, throbbing sensation, usually on one side of the head,
- Nausea or vomiting,
- Light and sound sensitivity
- Pain level rated as moderately severe,
- Tight, squeezing sensation, often around the entire head.
Possible Mechanisms of PTHA
How Pain Works
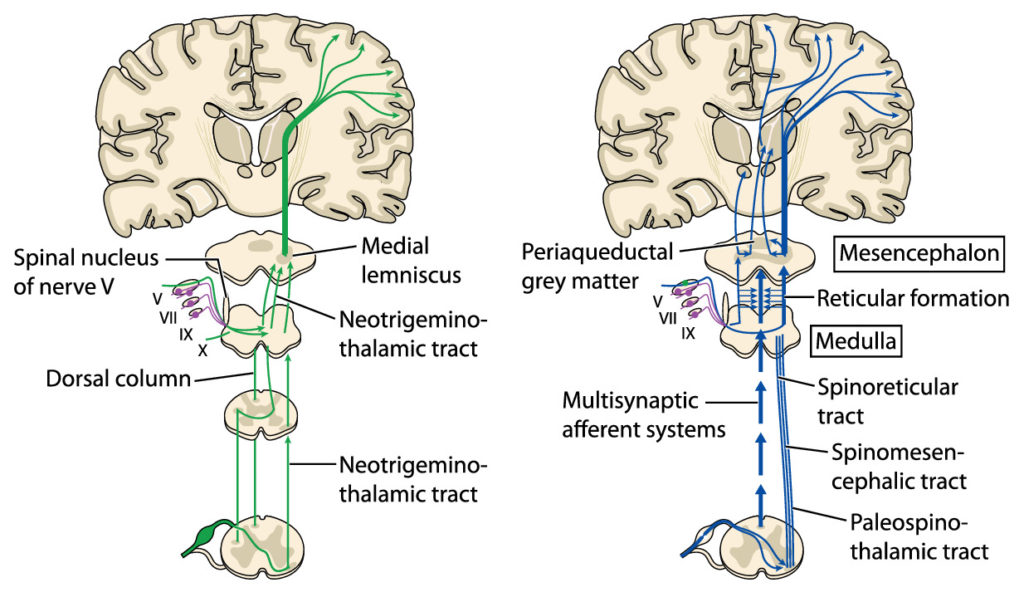
From all regions of the body where pain registers, pain pathways exist to carry pain signals to the brain for processing. These are called “ascending pathways” and consist of sensory nerves that start at the originating site of the pain and from there proceed to the spinal cord where they then connect to other sensory nerves that make up the rest of the pathway to the brain.
Once the pain signal arrives in the brain it is routed to the appropriate brain region to be assessed. This assessment occurs in what is called the “somatosensory cortex” where the pain sensation itself is initially “perceived.” This cortex is located at the top center of the brain, runs horizontally across the brain, and is responsible for processing all sensory input from the body (touch, visual, auditory, etc.), not just pain.
Pain processing involves multiple different areas within the somatosensory cortex depending on where in the body the pain signal came from. After this processing is complete, the brain then acts to counter the pain perception by initiating remarkable and delicately balanced countermeasures.7
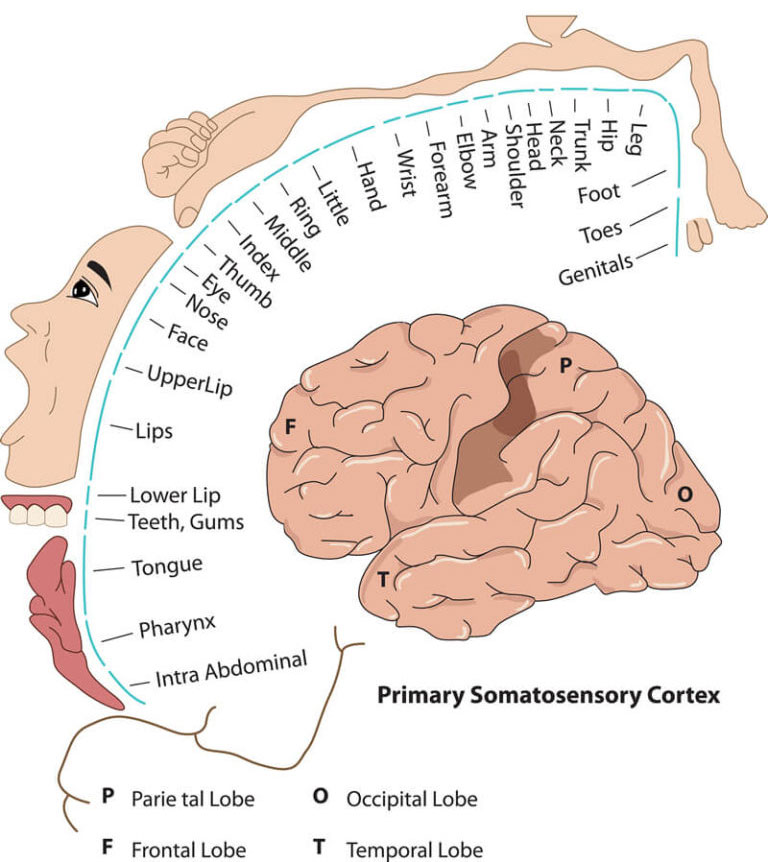
From the somatosensory cortex the healthy brain generates and sends “descending” signals through separate “descending pathways” that serve to inhibit the pain sensation. This pain modulation exists in the form of a descending pain modulating circuit with inputs that originally arise not only in the somatosensory cortex but in other areas of the brain (hypothalamus, amygdala, rostral anterior cingulate cortex), feeding then to the midbrain periaqueductal grey (PAG) with outputs from the PAG to the medulla, both of which are located in the brainstem. From there the descending signal travels down nerves to the place in the spinal cord where the ascending pain signal entered. At that location, certain neurotransmitters (chemicals) are then released from the descending pathway, namely serotonin, noradrenaline, and an endogenous opioid. These chemicals then react with the ascending pathway at that juncture to disrupt the ascending signal and thereby inhibit and control the pain. This is known as the “Gate Control Theory.”
In other words, brain-generated signals that inhibit pain have certain places of origin in the brain and certain pathways through the brain and down the spinal cord before they output and take effect. It is because of this endogenous inhibitory mechanism/system that a pain sensation eventually, under normal circumstances, subsides. If this system, however, becomes damaged or compromised, it is theorized that pain, otherwise inhibited, can become constant and/or chronic. Headache pain is no exception.8
Surprisingly, headache pain originates from tissues and structures that surround the brain, or surround the skull, or are located in the neck, but are not located within the brain itself. The brain has no sensory nerves that can give rise to the sensation of pain. Quite literally, a brain surgeon can cut the brain with a scalpel and the awake patient doesn’t feel anything. The brain merely processes pain signals that originate from sensory nerves located elsewhere.
Again, while the precise mechanisms of PTHA are unknown, there is consensus that such headaches are of two origins, peripheral (meaning originating outside the central nervous system) or central (meaning origination within the central nervous system), or both simultaneously. 7,8 The peripheral nervous system is comprised of the cranial nerves (except the optic nerve), the nerves that exit the spinal cord and innervate the body, and the autonomic nervous system. The central nervous system is comprised of the brain and spinal cord.
Peripheral Origin of Headache Pain
Many peripheral tissues if damaged or compromised have the potential to generate headaches. These may include intracranial arteries, dura mater (tough outer membrane covering the brain and spinal cord) and dural arteries, as well as extracranial structures such as bone, muscles, skin, ligaments, peripheral arteries, nerves exiting the cervical spine, cranial nerves, and deep fascia. While classified as peripheral, these structures located in the head and neck regions are not far removed from the brain itself. So, what causes these peripheral tissues to become painful antagonists in both acute and chronic phases?
Nociceptors
These peripheral tissues/structures, like most of the human body, are innervated with pain receptors called “nociceptors” (sensory nerves specialized to detect intense stimuli, like pain). Nociceptors are sensory receptors of the peripheral nervous system, and as previously mentioned, are connected to nerves that connect to other pain nerves in the spinal cord that then transfer the pain signal to the brain. This is referred to as “pain signaling” or “nociception.”8
Hypersensitivity
It is strongly held that PTHA of peripheral origin occurs when the ascending peripheral nerves carrying pain signals become hypersensitive through microscopic nerve damage caused in the traumatic event.7 This would involve damage to the nerves that surround the brain and skull, and those found in the neck. This damage results in “neurogenic inflammation,” an inflammation of adjacent nerves causing hypersensitivity to pain stimuli.9,10 As a result, pain signaling can be heightened and may produce a lasting, repetitive headache sensation. Also, vascular (blood flow) disruption in the head and neck regions from head trauma can occur with the same hypersensitivity result.8 While increased sensitivity of these nerves may resolve on their own, the sensitivity can become chronic leading to CPTHA.8
Cervical Spine Nerves
Direct injury or compromise to peripheral nerves in the neck may also contribute to or cause PTHA and CPTHA. Previously, the association between neck pain and PTHA was attributed solely to the presence of a whiplash injury and inflamed cervical nerve roots. That association remains valid and universally accepted. However, it is now known that if cervical damage occurs during the TBI event the nociceptor input from the cervical region reaching the trigeminal nucleus (area which receives ordinary sensations from the main three branches of the trigeminal nerve) might be a source of referred pain, and may also contribute to cranial mechanical hyperalgesia (increased sensitivity to pain) in individuals with PTHA.11 Stated another way, the head pain is the same but there may an additional source and causative origin to consider.
Cranial Nerves
The cranial nerves may be another possible peripheral source of PTHA and CPTHA. There are 12 cranial nerves that run on both sides of the skull and are vulnerable to direct force trauma. Damage to these nerve fibers, particularly trauma to the trigeminal vascular system, can provoke a cascade of cellular events in the area of the damaged site, ultimately changing the underlying spontaneous pain sensation. This can cause hypersensitivity and hyper-reactivity of pain nerves/neurons in this region.12,13
Central Origin of Headache Pain
Damage to certain central brain structures/tissues during TBI can lead to headache pain sourced from diffuse axonal shearing (breaking and twisting of neuron axons in the brain), alterations in cerebral blood flow, widespread brain neuron depolarization, and increased glutamate release causing what’s called “excitotoxicity.” These are injury-related processes that can cause brain damage on a widescale and would be considered central in origin. These processes once in motion can induce damage/alteration to various tracts, systems, and structures related to pain modulation, including damage/compromise to the all-important descending pain modulatory system/circuit and the supporting glial cells in the brain.
Central Origin of Headache Pain
Damage to certain central brain structures/tissues during TBI can lead to headache pain sourced from diffuse axonal shearing (breaking and twisting of neuron axons in the brain), alterations in cerebral blood flow, widespread brain neuron depolarization, and increased glutamate release causing what’s called “excitotoxicity.” These are injury-related processes that can cause brain damage on a widescale and would be considered central in origin. These processes once in motion can induce damage/alteration to various tracts, systems, and structures related to pain modulation, including damage/compromise to the all-important descending pain modulatory system/circuit and the supporting glial cells in the brain.
Descending Pain Modulatory System / Circuit
As mentioned, the spinothalamic tract is the extended pathway pain signals travel from nociceptors (where the pain signal originates), to and through the spinal cord, then ascend up and through the brain stem to the thalamus, which then sends the sensory impulses on to the appropriate regions of the somatosensory cortex for processing. The thalamus, located just above the brain stem, serves as the major “relay station” for all sensory information, including pain.14
To counter pain sensations certain other structures form a modulating/inhibiting pain circuit that inhibits pain signals as they ascend by a targeted release of endogenous opiates such as endorphins and enkephalins. This circuit consists primarily of brainstem structures such as the periaqueductal gray (PAG), the locus coeruleus (LC), the nucleus raphe magnus (NRM), the nucleus reticularis gigantocellularis (Rgc), and the amygdala (just above the brainstem) together with descending neurons. These structures through an interconnected circuit initiate signals the descending neurons carry down to the level of the spinal cord where the ascending pain signal first entered. When these descending signals arrive at the designated level, they activate to release opiates to moderate or stop the ascending signal.
If the brainstem structures that originate the pain inhibitory signals, or the descending tract, are compromised through brain trauma, these structures and/or the nerves within the tract can thus be “released” from their role in inhibiting the pain signal. Regarding headaches and stated in simpler terms, if the parts of the brainstem that send signals to stop headache pain are damaged from trauma, or if the tract itself is compromised, the pain inhibitor signals can cease to emit. This can cause trauma-related headaches, including chronic headaches 16,17to persist.
Glial Cells
A vast majority of cells in the brain and throughout the central nervous system are not nerve cells that transport impulses/signals, but rather what is called “glial cells” that serve in a vital support role. The four main functions of glial cells are: to surround neurons and hold them in place, to supply nutrients and oxygen to neurons, to insulate one neuron from another, and to destroy and remove the carcasses of dead neurons (cleanup)18. Animal models of TBI showing increases in pro-inflammatory factors and glial cell activation, and similar findings in humans after TBI, suggest that abnormalities in glial function contribute to conditions that initiate into a persistent neuronal dysfunction. Similarly, dysfunctional glial cells in the vicinity of damaged neurons in animal models of central pain were suggestive of secretion of pro-inflammatory agents, creating persistent glial inflammation and continued sensitization of neurons. It is therefore theorized that similar processes occur in the brain after TBI that underlie PTHA and CPTHA.19
Additional Contributing Factors
Individuals with TBI often present with various co-morbidities (the simultaneous presence of two or more chronic diseases or conditions) that may contribute to and/or maintain PTHA and CPTHA. For example, TBI and post-traumatic stress disorder (PTSD), an anxiety disorder that develops following exposure to a potentially life-threatening event, are known to co-occur. Research has found that PTSD symptomology was significantly higher in individuals with CPTHA compared to control groups.20,21
Post-traumatic Stress Disorder may also affect CPTH via increased levels of depression.22 Studies have shown that six months post rehabilitation discharge, the level of depression and anxiety in individuals after TBI correlated highly with headache density, and at 12-months the correlation between depression and headache density remained constant.20 Other symptoms might contribute to maintenance of CPTH, including sleep disturbances, memory difficulties, concentration disturbances, and nervousness and hyper-vigilance. Distress and personality changes documented in TBI subjects might also intensify CPTHA, which may in turn, perpetuate emotional problems that further exacerbate the pain.23
PTHA Treatment - A Multidisciplinary Approach
Treatment of PTHA and CPTHA depends on many factors. Generally, those with PTHA come to medical attention if, and when, the headache causes a significant pain, disability from, or inability to function or concentrate, all of which may lead to significant work loss and decreased social functioning. Many people will initially seek care from primary care physicians or sports medicine specialists if the injury is sports related. There may be many layers of physicians seen before a headache specialist or practitioner who is knowledgeable in PTHA is found. Ultimately, a multidisciplinary approach should be utilized. Not every therapy works for all. Often multiple treatment protocols are in place simultaneously.
Medicines
Medicines are available. However, despite the very high prevalence of PTHA there are surprisingly, no evidence-based guidelines for pharmacological treatment. Current pharmacological treatment of PTHA is based on acute or preventive medicines used for primary headache disorders (migraine, tension and cluster headaches), since PTHA often mimics their symptoms. This approach often results in poor treatment management and responses. That said, database research reveals the following medications for acute pharmacological treatment for treatment of PTHA are most utilized.24,25
Acute Pharmacology
- Ketorolac (analgesic/pain relief)
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Acetaminophen (analgesic/pain relief)
- Combination Drugs (Excedrin, Cafergot, Midrin)
- Lidocaine (anesthetic)
- Triptans (Naproxen, Imitrex, Frova, Amerge)
- Opioids (analgesics/pain relief) (last resort)
- Ondansetron (nausea/vomiting)
- Metoclopramide (nausea/vomiting)
- Prochlorperazine (nausea/vomiting)
Preventative Pharmacology
- Tricyclic antidepressants (amitriptyline, nortriptyline)
- Topiramate (reduces brain hyperexcitability
- Propranolol (beta blocker)
- Melatonin (hormone)
- Gabapentin (treats neuropathic pain)
- Flunarizine (calcium channel blocker)
Injection Procedures
Botox injections, epidural spinal injections, occipital nerve blocks, dry needling and acupuncture are in use to prevent and/or minimize certain types of headaches, mostly migraine. They are also in use for PTHA prevention and management. These are yet further examples of treatment procedures for primary headaches used to “treat” a secondary headache (PTHA) of completely different origin. These are efforts at symptom management, but certainly not curative.
Botox Injections
Botox injections were approved in 2010 by the U.S. Food and Drug Administration (FDA) for migraine treatment in adults that have a history of migraine headaches and headaches (including tension headaches) on most days (15 or more) in the month of which 8 are migraine. It is not approved for headaches of less frequency, or for cluster headaches. Botox is a neurotoxin and is believed works for migraine headaches because it inhibits chemicals (neurotransmitters) that carry pain signals to and within the brain. Botox serves as a roadblock in that pathway.26,27 Although this mechanism can reduce headache frequency and severity, it does not seem to change the underlying migraine condition.
Botox injections for headaches are given in 31 fixed sites in the face, head and neck. There may be as many as 30 to 40 injections given in a single setting. For optimal effect, they should be administered once every 12 weeks by a physician who is trained to give these shots rather than someone trained for merely wrinkles or other cosmetic uses.25 Adverse effects are very rare and are usually mild and transient, and rarely lead to abortion of therapy. Among the reported adverse effects were neck pain (4.3%), injection site pain 2.1%), eyelid droop (1.9%), and muscle weakness (1.6%) were most common.28
Epidural Spinal Injection
An epidural spinal injection is an injection of local anesthetic (numbing agent) and steroid medication (Kenalog or Depo-Medrol, anti-inflammatories) into the epidural space. The epidural space is located in the spine just outside the membrane that covers the spinal canal and nerve roots. Nerves travel through the epidural space in the cervical spine to the neck, upper extremities and head. Inflammation of these nerve roots can cause pain in these regions, including headaches. Epidural spinal injections (ESIs) are often used for PTHA given that often neck trauma is associated with TBI resulting in damage and subsequent inflammation of cervical nerve root endings. This damage, including the inflammation from the damage, can produce what’s called “cervicogenic” headaches. Cervicogenic headaches include whiplash-induced headaches, migraine without aura, migraine worsened by analgesic abuse, and tension-type headaches. They can also simply be related to degenerate conditions in the cervical spine.29
The cervical spine and associated muscular support compose a complex structure with many sites for generation of pain. Irritation of the cervical nerve roots at any point from their origin in the spinal cord to their paths to the occipital muscles can result in pain referred to the head, and neck, or upper extremity.30
Occipital Nerve Blocks
An occipital nerve block is a procedure that involves injecting at the base of the skull an analgesic and steroid compound into the greater and lesser occipital nerves, primarily for the treatment of headaches, including PTHAs. Patients generally receive 3 to 4 injections per year.
The occipital nerve is located at the base of the skull arising from the C2-C3 spinal nerves in the neck. It innervates the back of the scalp up to the top of the skull and the ears. Occipital nerve blocks have been found to be effective, by and large, for treatment of migraine and occipital neuralgia. In one study 562 patients with migraines who had greater occipital nerve blocks, 58 percent reported reduction of baseline pain scores.31 In another study, among 44 patients with occipital neuralgia, 95.45 percent said they were uncomfortable with their pain for at least 6 months, and only 16.67 percent of patients needed analgesics to cope with the pain after 6 months.32,33 Side effects are nominal and rare which include pain or irritation at the injection site, infection, stronger headaches, allergic reactions, dizziness, numbness and a small risk of nerve damage.
Dry Needling
Dry needling involves inserting long or small-filament needles deep into myofascial “trigger points” of effected muscles. Myofascial trigger points or simply trigger points, also known as knots in muscle tissue, are a group of muscle fibers which have shorted after activation or injury but have not been able to lengthen back to a relaxed state. A myofascial trigger point is characterized by the development of a sensitive nodule in the muscle.34 This occurs when the muscle fibers become so tight they compress the capillaries and nerves in the area.35 With this tightening the muscle is unable to move normally, obtain a fresh blood supply containing oxygen and nutrients, or flush out additional acidic chemicals. In addition to the nodule itself, the remainder of the muscle tightens to compensate.35 This causes pain at the site and can ultimately cause headaches. Dry needling assists with decreasing local muscular pain and improving function through restoration of a muscle’s ability to lengthen normally by releasing the trigger point.35
When the needle is inserted into the center of a trigger point, blood pools around the needle triggering the contracted muscle fibers to relax by providing those fibers with fresh oxygen and nutrients, as well as flushing away any additional acidic chemicals. This, in turn, leads to the decompression of the local blood and nerve supply. Dry needling does not involve the injection of any substance and is typically administered by a physical therapist. Scientific studies on dry needling are limited, though the body of resource is growing. So far, some research has been positive.36
Acupuncture
Acupuncture is not the same as dry needling. Acupuncture is practiced by tens of thousands of licensed acupuncturists. Expert acupuncturists train from three to four years. The training includes both instruction in use of needles and instruction in diagnosing conditions. Practitioners have direct supervision from another senior or expert practitioner. In addition to this training, acupuncturists must undergo testing from a national board of examiners and continue to take instructional courses each year to maintain their license. The American Medical Association recognizes acupuncture as a medical treatment, and some insurance companies may cover treatment costs.
The fundamental belief of acupuncture is that illness is the result of blocked or interrupted chi. Chi provides your body with healing energy. Acupuncture seeks to remove these blockages and return your energy flow to a state of balance and is used to treat several types of pain. A 2017 study found that regular acupuncture could help to prevent migraines37 and a 2012 review of migraine studies found that acupuncture was at least as effective at easing migraines symptoms as other classic treatments, including with longer lasting effects, lower medication use, and with fewer serious complications.38
Summary
Post-traumatic headaches remain a debilitating symptom of brain injury with current treatment options limited, for the most part, to symptom management. This is because, like all other lasting symptoms of brain injury, medical science has yet to discovery how to heal certain damaged microscopic tissues. But, the medical challenge with this PTHA and CPTHA at this stage is even more fundamental. We don’t fully know exactly which compromised brain structures and altered systems are producing this pain, nor how. We have working theories, models and reported clinical results, but not much more. Seems until these elementals are known and understood, no true medical breakthrough can occur. That said, there is reason for optimism.
There are certainly credible, data-based theories with effective models in use in the practicing theater that are helping those in pain and represent notable progress. Additional treatment protocols are coming online and increased efficacy has been demonstrated in existing options. Multidisciplinary approaches are in constant refinement. New investigative tools and techniques are available now unlike ever before. Our body of knowledge and understanding is constantly expanding. As a result of these and other positive events, the quality of life for more and more patients is clearing improving.
However, new discoveries in brain function, healing propensities, and more effective medical pain management will likely not be the full answer to post-traumatic headaches. This unexplored frontier appears broader than that. The key to unlocking the enigma of this head pain likely lies in unraveling the mystery of pain itself. We know the sensory nerves involved in perceiving pain initially and the pathways pain signals take to the brain. But how the brain then routes, defines, catalogs, processes and thereafter responds to all pain stimulus, not just headache pain stimulus, is not known with certainty. It is also largely unknown why and how certain sensory peripheral nerves become hyperactive and hypersensitive through apparent inflammation and persist in that state, causing headache and chronic headache pain sensation. What is known is somewhere, somehow a breakdown in the overall headache pain management system occurs in association with the traumatically damaged brain and neck. When the riddle of that breakdown is solved, then a true revolution in the management and cure of headache pain, and perhaps all chronic pain, will likely follow.
– Charlie Waters
References
1Packard RC. Chronic post-traumatic headache: associative with mild traumatic brain injury, concussion, and post-concussive disorder [Review] CURR PAIN HEADACHE REP. 2008; 12:67-73
2Baandrup L. Jensen R. Chronic post-traumatic headache-a clinical analysis in relation to the International Headache Classification 2nd Edition. Cephalalgia 2005; 25:132-8.
3Lenaerts ME. Post-traumatic headache; from classification challenges to biological underpinnings [Review]. Cephalgia. 2008; 28 (suppl 1):12-15.
4Couch Jr. Lipton RB, Stewart WF, Scher A1. Head or neck injury increases the risk of chronic daily headaches; a population -based study. Neurology 2007:69:1169-1177.
5Ahmed F, Headache Disorder: differentiating and managing the common subtypes, British Journal of Pain, 2012 6(3) 124-132
6Lucas S, Characterizing and Management of Headaches after Mild Traumatic Brain Injury, 2015.
7Basbaum AJ, Bautistas D, Julius D, Cellular & Molecular Mechanisms of Pain Cell, 2009 Oct 16:139(2):287-284. Author manuscript.
8Basbaum AJ., Jessell TM. The perception of pain. IN: Kandel ER, Schwartz JH, Jessell editors. Principal of neural sciences, 4th edn. New York, McGraw-Hill; 2000. p 472-91.
9Elliott MB, Oshinsky ML, Amenta PS, Awe 00, Jallo JI., Nociceptive neuropeptide increases and periorbital allodynia in model at traumatic brain injury. Headaches. 2012; 52:966-84.
10Perez-Polo JR, Rea HC, Johnson KM, Porsley MA, Unabia GC, XU GJ, et al, Inflammatory consequences in a rodent model of mild traumatic brain injury. J Neurotrauma. 2013; 30:727-40.
11Bartsch T. Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002; 125:1496-509.
12Devon M, Govrin-Lippermann R. Rappaport ZH. Mechanism of trigeminal neuralgia; an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J. Neurosur. 2002; 96:532-43.
13Luo DS, Rang T. Zuo CX, Zuo ZF, Li H, WU SX, et al. An animal model for trigeminal neuralgia by compression of the trigeminal nerve root. Pain Physician. 2012; 15:187-96.
14Sherman SM, Guillery R. Functional organization of thalamotic relays. Journal of Neurophysiology. 1996; 712-713 [PubMed: 1358041
15Lenz FA, Tasker RR, Dostrovsky JO, Kwaw HC, Gorecki J, Hirayama T, et al. Abnormal single unit activity recorded in the soma sensory thalamus of a quadriplegic patient with central pain. Pain. 1987; 31:225-36.
16Wasner G, Lee RB, Engel S, McLaclan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008; 131 (Pt 9):2287-400.
17Tracy I. Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007; 55(3): 391. [PubMed: 17678852]
18Jessen KR, Mirsky R (August 1980) “Glial cells in the enteric nervous system contain glial fibrillary acidic protein.” Nature 286 (5774):736-7. doi:10. 1038/28673a0 [PubMed].
19Xie YE. Glial involvement in trigeminal central sensitization. Acute Pharmacol Sin. 2008; 29:641-5. doi:10.1111/J. 1745-7254. 2008.0081.X. [PubMed].
20Bryant RA, Marosszeky JE, Crooks J. Baguley IJ, Gurka JA. Interaction of post-traumatic stress disorder and chronic pain following traumatic brain injury. J Head Trauma Rehab. 1999; 14:588-94.
21Hoge CW, McGurk D. Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med 2008; 358:453-63.
22Asmundson GJG, Coons MJ, Taylor S, KATZ J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. C. J Psychist 2002; 47:930-7.
23Carroll LJ, Cassidy JD, Peluso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO collaborating center task force on mild traumatic brain injury. J Relabel Med. 2004; 43(suppl): 84-105.
24Smith, J., Acute treatment of migraine in adults, 2019, Up To Date.
25Azize, G., Mustafa, E., Prophylactic Treatment of Migraine, Noro Psikiyatr ARS, 2013 Aug; 50 (supp 1): 530-535
26Aurora, S., Dodick, D., Dienen, H., DeGryse, R., Turkel, C. Lipton, R. et al (2014) OnabotulinumtoxinA. for chronic migraine; efficacy, safety and tolerability in patients
27Runnel, A. (2015) The long journey of botulinum neurotoxins into the synapse. Toxicon 107:9-24.
28Bluefield, A., Silberstein, S., Odrick, D., Aurora S., Turkel, C. and Binder, W. (2010) Method of injection of OnabotulinumtoxinA for chronic migraine: safe, well-tolerated, and effective treatment paradigm.
29Pukis, J., Welch, M. Dotard, S. and Foster, K. (2000) Capsaicin-Stimulated release of substance P from cultured dorsal root ganglion neurons: involvement of two distant mechanisms. Brioche Pharmakoi 59:1403-1406.
30Bogduk, N: The anatomical basis for cervicogenic headache. J. Manipulative Ther 1992, 15:67-70.
31Kemp WJ, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int.. 2011; 2:178 [PubMed]
32Allen SM, Mookadam F, Cha SS, Freeman JA, Starling AJ, Mookadem M. Greater Occipital Nerve Block for Acute Treatment of Migraine Headache: A Large Retrospective Cohart Study. JAM Board Fam Med 2018 March-April; 31(2):211-218 [PubMed]
33Juskys R Sustickas G. Effectiveness of treatment of occipital neuralgia using the nerve block technique: a prospective analysis of 44 patients. Acta Med Litu 2018; 25(2):53-50 [PubMed]
34Simons, Travell, Simons, Myofascial Pain & Dysfunction 1999.
35McPortland J, Simons D, Myofascial Trigger Points: Translating Molecular Theory into Manual Therapy, Journal
36Dunning J., Butts R., Peneoul T T., Dry needling: a literature review with implications for clinical practice guidelines, Phys Ther Rev. 2014, Aug:19(4):252-265 of Manual & Manipulative Therapy, Volume 14, 2006.
37Zhao L, Chen J, Li Y, The Long-term Effect of Acupuncture for Migraine Prophylis, JAMA Inten Med. 2017; 177(4): 508-515.
38Molsbeyer A., The role of acupuncture in the treatment of migraine, CMAJ: Canadian Medical Association Journal


